"I'm Rh negative an had to get a shot while pregnant, why?"
Rh-negative Mothers and the Importance of Rh Immune Globulin Shots
Overview of Rh-Negative Blood Type:
- Rh-negative blood type is rare, found in only about 15% of the global population.
- It becomes particularly significant in pregnancy when an Rh-negative mother carries an Rh-positive baby, potentially leading to Rh incompatibility.
Rh Incompatibility Explained:
- Occurs when an Rh-negative mother is exposed to Rh-positive blood from her baby during pregnancy or delivery.
- This exposure can lead the mother's immune system to produce antibodies against the Rh factor.
- In future pregnancies, these antibodies can attack the red blood cells of an Rh-positive baby, a condition known as hemolytic disease of the fetus and newborn (HDFN).
Hemolytic Disease of the Fetus and Newborn (HDFN):
- HDFN can cause anemia, jaundice, brain damage, or even death in severe cases.
- It's a condition where the mother's antibodies destroy the baby's red blood cells.
Prevention with Rh Immune Globulin (RhIg or RhoGAM):
- RhIg is a blood product containing antibodies against the Rh factor, preventing the mother's immune system from producing harmful antibodies.
- Recommended for Rh-negative mothers to prevent Rh incompatibility and HDFN in future pregnancies.
Administration of RhIg:
- Given around 28 weeks of pregnancy and within 72 hours after delivery if the baby is Rh-positive.
- Also recommended after procedures that may mix maternal and fetal blood, such as amniocentesis or miscarriage.
Safety and Side Effects:
- While generally safe and effective, RhIg may cause side effects like pain or swelling at the injection site, fever, or allergic reactions.
- It's important for Rh-negative mothers to discuss the benefits and risks of RhIg with their healthcare provider and report any adverse reactions.
This comprehensive approach to managing Rh incompatibility significantly reduces the risks associated with HDFN, ensuring safer pregnancies for Rh-negative mothers and their babies.
Anti-D Antibody Titers
What Are Anti-D Antibodies?
- Anti-D antibodies indicate that a mother has been sensitized to the Rh factor (D antigen). This sensitization can occur through previous pregnancies, blood transfusions, or exposure to Rh-positive blood.
- The presence of these antibodies can lead to hemolytic disease of the fetus and newborn (HDFN) in future pregnancies.
Monitoring HDFN Risk with Antibody Titers:
- Healthcare providers use antibody titers to measure the concentration and potency of the mother's Anti-D antibodies during pregnancy.
- These blood tests are crucial for assessing the risk of HDFN and determining the appropriate course of treatment.
Interpreting Antibody Titer Results:
- Low Titers: May indicate a lower risk of HDFN. Close monitoring of the pregnancy may be sufficient.
- High Titers: Suggest a higher risk of HDFN. This situation may require more aggressive interventions, such as:
- Early delivery to prevent severe complications.
- Intrauterine blood transfusions to treat fetal anemia.
- Exchange transfusions after delivery to manage severe HDFN.
Additional Monitoring for the Baby's Health:
- Alongside antibody titers, healthcare providers also employ:
- Ultrasounds: Regular scans to monitor the baby's development and detect any signs of anemia or hydrops.
- Amniocentesis: A diagnostic test to assess the severity of anemia and other complications in the fetus.
This comprehensive approach allows for the early detection and management of potential complications associated with Anti-D antibodies, aiming to ensure the health and well-being of both the mother and the baby throughout the pregnancy and after birth.
Intrauterine Transfusions for HDFN
Exchange Transfusion for HDFN
An exchange transfusion is a medical procedure that may be used to treat severe cases of hemolytic disease of the fetus and newborn (HDFN) caused by Rh incompatibility between the mother and fetus. During this procedure, a newborn's blood is gradually replaced with Rh-negative donor blood in order to remove the Rh-positive red blood cells that have been attacked by the mother's antibodies.
The procedure involves placing catheters into the baby's umbilical vessels or other blood vessels, and gradually removing small amounts of the baby's blood and replacing it with donor blood.
The goal of the exchange transfusion is to reduce the levels of bilirubin, a yellow pigment that can build up in the baby's blood and cause jaundice, as well as to remove the Rh-positive red blood cells that have been attacked by the mother's antibodies.
Exchange transfusion may be necessary when the baby's bilirubin levels are very high, or when the baby is showing signs of severe anemia, such as low oxygen levels, fast heartbeat, or lethargy. The procedure can help to prevent or treat the complications associated with severe HDN, such as brain damage or organ failure.
Kleihauer-Betke Test
Purpose and Importance:
- The Kleihauer-Betke (KB) test quantifies fetal-maternal hemorrhage (FMH), the mixing of fetal and maternal blood during pregnancy, childbirth, invasive procedures, or trauma.
- It's particularly crucial for Rh-negative mothers to prevent the production of antibodies against the Rh factor, which could lead to hemolytic disease of the fetus and newborn (HDFN) in subsequent Rh-positive pregnancies.
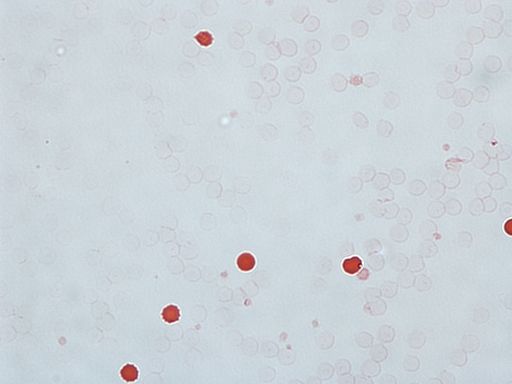
A KB stain positive for fetal blood, noted with the dark red cells.
Test Procedure:
- Staining: A maternal blood sample is stained with a specific dye that highlights fetal red blood cells.
- Microscopic Examination: The stained sample is examined under a microscope to calculate the percentage of fetal cells in the maternal blood.
- Determining RhIg Dosage: The KB test results help determine the appropriate Rh immune globulin (RhIg) dose needed after a sensitizing event to prevent HDFN.
Associated Tests:
- Fetal Bleed Screen: A preliminary, rapid test to detect if fetal and maternal blood have mixed. It identifies the presence of RhD positive red cells in an Rh-negative mother's blood.
- Determining Further Action: A positive Fetal Bleed Screen typically leads to a KB test to decide if the standard RhIg dose suffices or if additional doses are required, considering one RhIg vial can protect against up to 30mL of RhD positive whole blood.
Significance for Rh-Negative Mothers:
- The KB test, alongside the Fetal Bleed Screen, plays a vital role in managing Rh-negative pregnancies, ensuring the well-being of both mother and future pregnancies by accurately adjusting RhIg therapy to prevent HDFN.